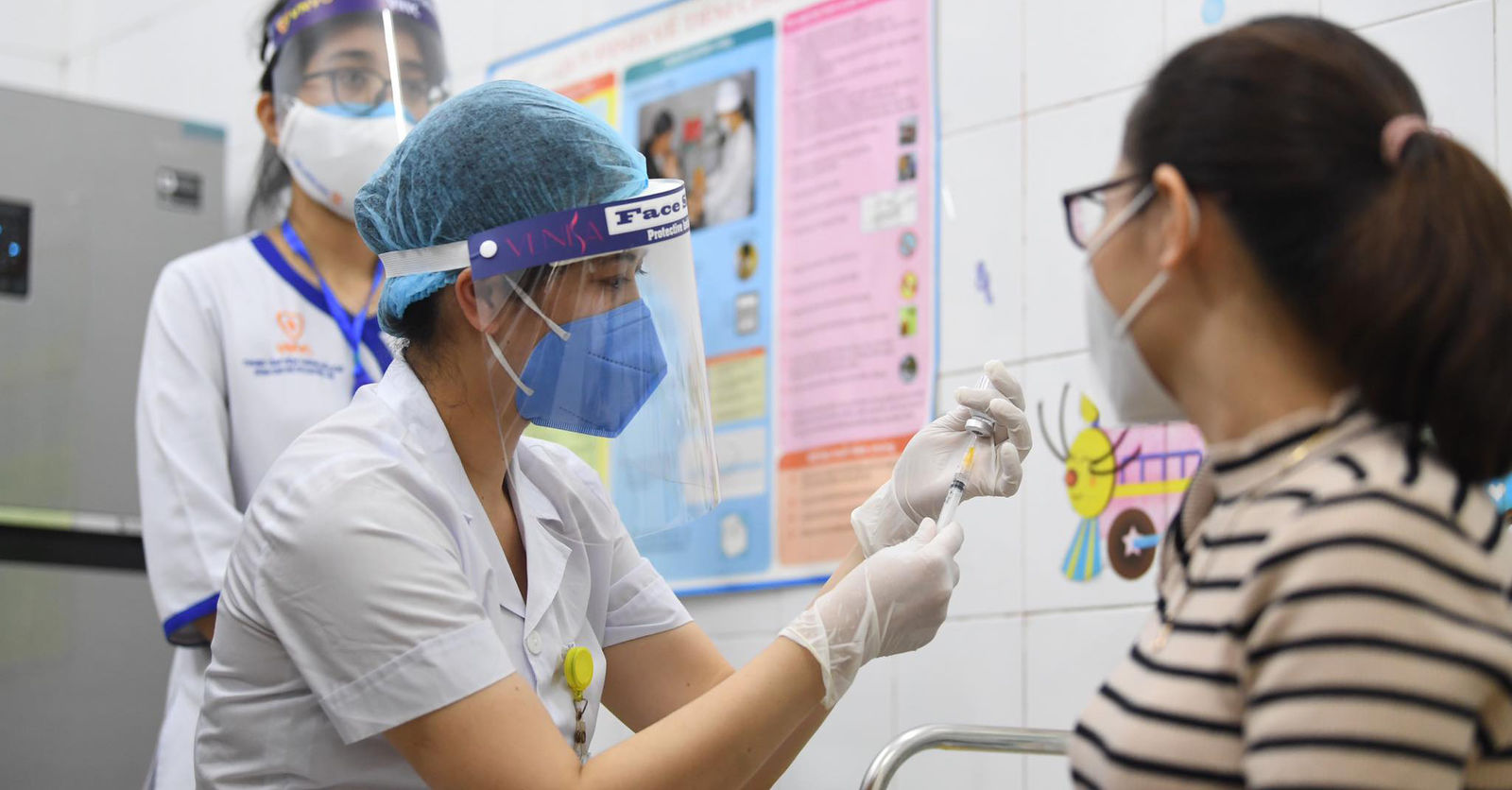
A second COVID-19 candidate vaccine has officially entered the human trial phase.
On January 21, the Nha Trang-based Institute of Vaccines and Medical Biologicals (IVAC) announced that its COVID-19 vaccine candidate, named Covivac, will start being administered to human volunteers, reports Tuoi Tre. Covivac is the product of a collaborative effort by IVAC, the National Institute of Hygiene and Epidemiology, and the Hanoi Medical University.
The vaccine is Vietnam’s second to enter human trials; the first, created by Nanogen, started the process back in December last year. Covivac, however, will cover a wider range of human subjects from 18 to 75 years old.
According to IVAC Director Dương Hữu Thái, preclinical trials of the vaccine in India, the US and Vietnam demonstrated sufficient efficacy and safety to move to human trials, which will be undertaken in two phases.
The first, from January to April, has 120 participants who will receive a range of different dosages. The second runs from July to August with 300 participants who will receive two different levels of vaccine to determine the most effective one.
"It is developed based on embryonic production technology that is also used to produce seasonal influenza vaccines in Vietnam,” Thái added.
Covivac can be kept at temperatures of 2–8°C, making it easier for storage. In comparison, Pzifer’s vaccine has to be refrigerated between -80°C and -60°C. Production facilities operated by IVAC are capable of producing up to 30 million doses of Covivac a year.
Over the past year, Vietnam has also amped up COVID-19 testing power. At the moment, there are 148 laboratories across the country capable of running Realtime RT-PCR tests at over 51,000 samples a day. Since the beginning of the coronavirus epidemic in Vietnam last January, Vietnam has tested a total of 1.5 million samples for SARS-CoV-2.
[Photo via VNA]